Abstract
Follicular lymphoma (FL) is typically indolent, but despite treatment, relapses and transformation to higher grade disease are common. Ionizing radiation therapy (RT) is a guideline-preferred treatment for localized disease, and FL can be exquisitely radiosensitive, as demonstrated by the potential for complete response (CR) to very low dose RT (VLDRT, 4Gy). Clinicodemographic factors associated with durable FL response to RT are crude and though there are many predictive models of response to rituximab or chemotherapy, there are no known genetic markers of FL radiosensitivity. We sought to define genetic signatures of radiosensitivity in FL patients.
We analyzed a single institution database of FL patients (all stages) treated with RT between 2005-2021 and identified 98 patients with 101 tumors each having preexisting MSK-IMPACT, a targeted exon sequencing panel assaying up to 505 genes. We also identified an additional validation cohort of 12 patients who did not have pre-existing mutation data for which we performed de novo MSK-IMPACT. We used hierarchical clustering with Ward criterion, and using scree plot analysis, identified an optimal number of gene alteration clusters. Using logistic regression and Kaplan Meier analysis, we associated the presence of gene alterations with best response to RT between 2-6 months using Lugano criteria and local progression (LP), respectively. We used LASSO Cox regression to derive a parsimonious model relating gene alterations to local progression. Lastly, we mined gene interaction networks related to genetic interactions, RT response, and gene regulation to identify dysregulated gene networks.
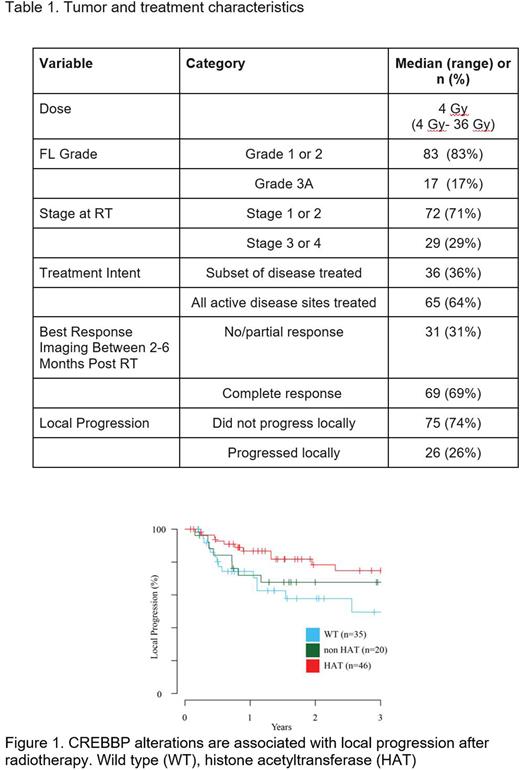
The median RT dose was 4Gy (range: 4-36Gy, with 57 tumors (56%) receiving 4Gy. 83 tumors (83%) were grade 1-2. 72 (71%) tumors were early-stage at the time of RT, and 65 (64%) tumors were the only active site of disease in a patient at the time of treatment. 69 (69%) tumors had a CR, and with median follow-up of 25 months, 26 (26%) had local progression after RT (Table 1). FL grade was not associated with alterations in any gene, but alterations in MEF2B and BCL2 were associated with more advanced stage at RT (p=0.004 and p=0.017, respectively). The most prevalent mutations were CREBBP (65%), KMT2D (51%), TNFRSF14 (50%), STAT6 (27%), EZH2 (25%), BCL2 (24%), IRF8 (23%) and FOXO1 (21%), many of which are known epigenetic transcription regulators. We identified 5 clusters of altered genes with most clusters having CREBBP alterations, but one BCL2 altered cluster without CREBBP alterations that had significantly shorter LP after RT (log-rank p=0.02). From LASSO regression modeling, CREBBP, STAT6, B2M, TNFRSF14, HIST1H1E, TP53, MEF2B, FOXO1 and BCL2 were retained, with CREBBP and BCL2 having the largest effect sizes, indicating that alterations of these two genes make the largest contribution to observed LP after RT. Coding mutations occurring within CREBBP were associated with increased odds of CR (Odds ratio: 3.02 (95% CI: 1.25-7.33, p=0.014)) and reduced LP (median LP for mutations 136 months vs wild-type 31 months, log-rank p=0.02). Because CREBBP has multiple functional domains, including a histone acetyltransferase (HAT) domain responsible for transcriptional regulation, we further sought to contextualize the alterations within CREBBP. HAT domain mutations show a significant association with reduced LP risk after RT (Hazard ratio:0.37 (95% CI: 0.15-0.89, p=0.019)) (Figure 1). This effect was even more pronounced for the cohort of tumors receiving VLDRT of only 4 Gy (n=56) (log-rank p=0.05), and we confirmed this effect in our validation cohort (log-rank p=0.05). Lastly, we identified subnetworks of interconnected genes that are enriched in apoptosis and the immune system regulation.
Alterations in the HAT domain of CREBBP, which likely result in a loss of function, are associated with better response to RT. Genetically defined cluster signatures can be further explored to understand the additive effects of alterations in addition to CREBBP on treatment response. Additionally, as histone acetylation by CREBBP is implicated in the radiosensitivity of FL, it is possible that agents targeting epigenetic processes may have a role in increasing radiosensitivity during FL treatment. The genetic landscape of FL patients being treated with radiotherapy, especially VLDRT, may be incorporated into clinical decision making to improve patient and dose selection for RT.
Disclosures
Lebow:Oncia Technologies, Inc: Current equity holder in publicly-traded company. Joffe:Abbvie: Honoraria; Beigene: Honoraria. Dogan:Incyte: Consultancy; Peer View: Honoraria; Loxo: Consultancy; EUSA Pharma: Consultancy; Physicians' Education Resource: Consultancy, Honoraria; Seattle Genetics: Consultancy; Takeda: Other: Research Funding; Roche: Other: Research Funding. Imber:GT Medical Technologies: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal